An interdisciplinary team of Texas A&M University scientists led by Texas A&M chemist Lei Fang has developed a novel solution-processable, solvent-resistant polymer capable of maintaining its structure in the face of extreme conditions and being converted into thin film, fibers or virtually any shape desired for use in any number of potential industries and applications.
“This is a class of polymer called a conjugated ladder polymer,” Fang said. “It has a promising future of applications because it has extremely good stability in combination with advantageous electronic and mechanical properties over conventional polymers as well as conventional organic materials in general.”
The findings from the team’s three-year research project were published earlier this month in the Elsevier journal Chem. The work was funded by grants from the American Chemical Society Petroleum Research Fund and the Texas A&M Energy Institute and conducted in part using the resources of the Advanced Photon Source through the U.S. Department of Energy-funded Argonne National Laboratory.
Fang, a faculty member since 2013 in the Texas A&M Department of Chemistry who also holds a joint appointment in the Department of Materials Science and Engineering, is head of a multidisciplinary research group studying functional organic materials with electronic, thermal or photo-activity in search of applications associated with electronics and energy conversion/storage.
Fang notes that, although there has been big demand in the research community for such a revolutionary type of material, it hadn’t yet been well-developed because of the multiple challenges involved — first and foremost, making a desired polymer with more than one strand of bonds along the chain. His team, composed of faculty and graduate students from Texas A&M’s Departments of Chemistry, Aerospace Engineering and Materials Science and Engineering, rose to that occasion and then some, successfully developing one that is highly efficient, cost-effective and scalable.
“There are reports about making these types of materials, but most of them either require very tedious synthesis or can only be made in small quantities, such as a couple tens of milligrams,” Fang said. “We can make this material in multi-gram scale, and we haven’t reached the limit yet. If we want to try, we probably can make it in kilograms.”
But if it was easy, anybody could and would have done it. Fang says no one had for one simple yet incredibly complex reason: strong interactions between molecules that make these materials highly insoluble. Also, because the molecular structure is so rigid and so flat, the molecules prefer to aggregate to each other, making it very difficult to dissolve or melt it in order to process it into another form.
“Rigid molecular backbones and strong intermolecular interactions are crucial to a wide range of functional properties of solution-processable polymeric materials, such as stability under extreme conditions, mechanical strength and electronic conductivity,” added Dr. Yang Zou, a postdoctoral scholar who joined Fang’s lab in November 2013 and took on this research problem as his first project. “Ladder polymers possessing these features, however, have remained relatively underdeveloped because of the difficulty in precision chemical synthesis and solution processing.”
Fang’s group succeeded, thanks to a simple chemical modification they previously had demonstrated as effective in another system. They used the same technique here to intentionally block the key function group preventing solubility, thereby paving a clear path to the eventual breakthrough.
“Normal polymers are usually processed into thin films or fibers so that they can be used to fabricate different types of useful things,” Fang said. “In this case, we were able to address this problem by using a very simple reversible chemical modification. We converted this insoluble material into a soluble intermediate that we could then handle and manipulate however we want. And because this process is reversible, we can regenerate the original state by just simple heating. We demonstrated that we can process it into thin film, but in principle, we can make it into fibers or any shape we want.
“I think this will have a big impact. I can see many other peers who would probably use this method.”
While the dichotomy isn’t lost on Fang, he says it’s a balanced contradiction that succeeds at any extreme, including some of the most corrosive substances — boiling ones, at that.
“It is really, really robust and extremely stable against very aggressive solvents,” Fang said. “For example, we soaked it in boiling organic solvents like chlorobenzene. It’s also highly stable against concentrated hydrochloric acid. Exposing this thin film to such aggressive conditions doesn’t change its thickness at all. It doesn’t swell, either.”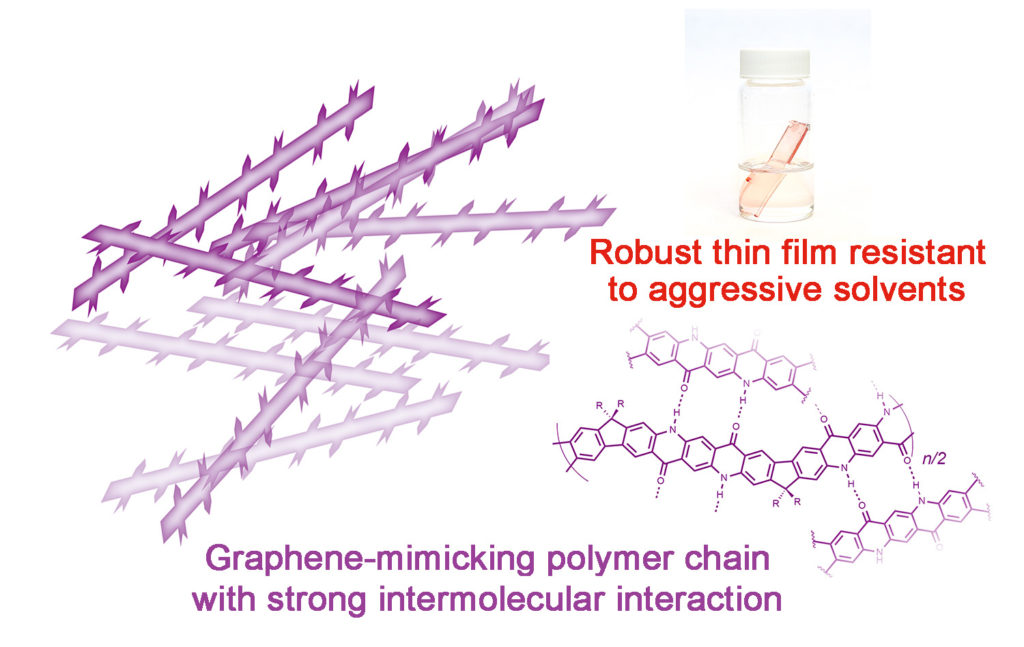
Unflinching durability and endurance notwithstanding, Fang says the super polymer’s most impressive quality lies in its limitless potential. When it comes to its applied future, he says the sky’s the limit — or more accurately, merely one of them, thanks to one other material advantage the team equipped it with as part of the synthesizing process.
“Because the material itself has this built-in, we call it graphitic type of structure — meaning that it mimics graphite — it’s a good precursor to be processed into high-performance carbon material, like carbon fibers,” Fang said. “Carbon fiber is a very strong and light material used in fancy cars, in space and even in golf clubs. So this material would probably also have a promising future in that direction of application.”
For additional insight into the discovery, see the complete first-person Chem article from Zou, now an assistant professor of chemical engineering at Guangong University of Technology. He and Texas A&M chemistry graduate student Xiaozhou Ji are joint first authors on the team’s paper.
“One of the most valuable gifts from my postdoctoral research has been an interdisciplinary scope,” Zou tells Chem in his article. “Without my labmates’ ideas on Boc protection, I would not have been able to achieve solution-phase characterization and processing of this unique class of polymer.
“In addition, we had the privilege of working with colleagues in different disciplines in order to finish this comprehensive project. Dr. Mohammad Naraghi’s group in the Department of Aerospace Engineering at Texas A&M University viewed our project from a different perspective outside our comfort zone, so they were able to give incredibly valuable insight.”
To learn more about Fang and his research group, visit http://www.chem.tamu.edu/rgroup/fang/.
https://today.tamu.edu/2016/11/15/rising-star-chemist-lei-fang-earns-nsf-career-award/
# # # # # # # # # #
About Research at Texas A&M University: As one of the world’s leading research institutions, Texas A&M is at the forefront in making significant contributions to scholarship and discovery, including that of science and technology. Research conducted at Texas A&M represented annual expenditures of more than $866.6 million in fiscal year 2015, ranking Texas A&M in the top 20 of the National Science Foundation’s Higher Education Research and Development survey (2015). Texas A&M’s research creates new knowledge that provides basic, fundamental and applied contributions resulting, in many cases, in economic benefits to the state, nation and world. To learn more, visit http://research.tamu.edu.
-aTm-
Contact: Shana K. Hutchins, (979) 862-1237 or shutchins@science.tamu.edu or Dr. Lei Fang, (979) 845-3186 or fang@chem.tamu.edu
This story was originally posted on science.tamu.edu