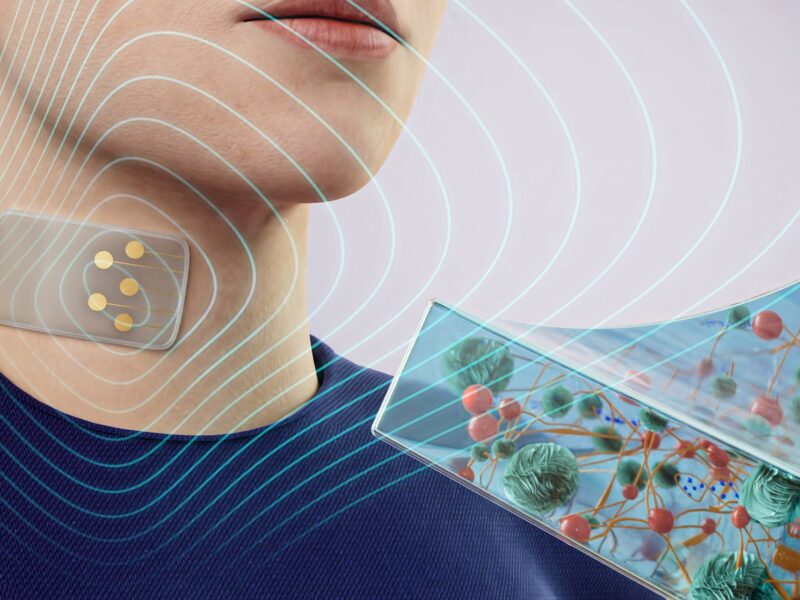
Texas A&M Researchers To Study Blood Pressure During Sleep

High blood pressure is the single biggest risk factor for heart disease, stroke and other health problems. The only way to know if you’re at risk is to have it checked often. While one in three American adults has high blood pressure, about 20 percent of people are unaware that they have it because it is largely symptomless. Researchers at Texas A&M University hope to help remedy this with a wrist-worn system that monitors blood pressure during sleep.
Roozbeh Jafari, a professor in the biomedical engineering, computer science and engineering, and electrical and computer engineering departments, and his team have received a $3.6 million grant from the National Institutes of Health (NIH) to create a system a user can wear all night while they sleep for constant readings.
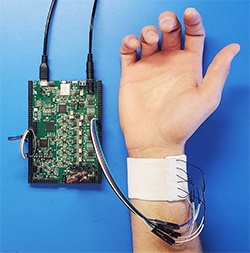
Blood pressure is the force of blood pushing against the walls of arteries as the heart pumps blood. High blood pressure, also referred to as hypertension, is when that force is too high and begins harming the body. If left untreated, it will eventually cause damage to the heart and blood vessels. Regular blood pressure monitoring systems use a mercury-based (or the digital equivalent) inflatable cuff-based sphygmomanometer. Many factors can affect blood pressure readings like caffeine, stress and exertion, and most people do not have theirs checked outside of a doctor’s office.
“There is a significant need to understand how blood pressure fluctuates throughout the day and night,” Jafari said. “Nobody knows that, and there’s really no technology that can capture this continuously.”
Jafari said there is a value to measuring blood pressure continuously in the natural context of the user’s environment, especially during sleep, without being disturbed by the instrument. But the nature of the current cuff device allows for only infrequent measurements and its somewhat invasive nature and associated discomfort prohibits additional nocturnal measurements.
The objective of this research, a collaborative project with the Yale School of Medicine, is to create an unobtrusive, wrist-worn, cuffless blood pressure monitor for measurement and identification of nocturnal nondipping hypertension (when there’s a smaller decrease in nocturnal arterial blood pressure). The research, which began initially about three years ago, includes extensive validation with state-of-the-art ambulatory blood pressure monitors at nighttime in the presence of varied treatment models.
Their proposed technology will be able to provide a wealth of information to physicians, help identify certain short-term dynamics and variations of blood pressure, and allow effective monitoring of response to medication, among other things.
“Nighttime blood pressure is actually a really good indicator for the health of the cardiovascular system,” Jafari said. “At nighttime, typically the body itself shows its true behavior. You and I can get stressed out, we can have a relaxing time and you could be very active physically, right? All those specific stimuli will impact the blood pressure. But at nighttime, you don’t have that, so you’re sort of having a baseline. Another significance of nighttime blood pressure is that you don’t have a lot of movement. The movements themselves introduce a significant amount of noise and challenges with respect to capturing clean signals.”
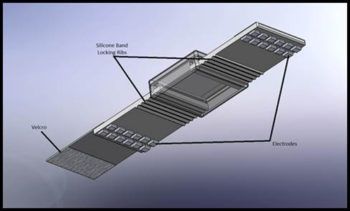
Jafari said nocturnal measurements would provide additional prognostic value in identifying risks, but despite these benefits, no wearable, noninvasive device for continuous blood pressure monitoring exists on the market simply because none have been reliable enough to be considered clinical grade.
The researchers set about developing a robust and reliable blood pressure monitor that uses bioimpedance sensors (a measure of how well the body impedes electric current flow) and for the first time, demonstrates clinical-grade reliability. They use sensors that measure pulse-wave velocity (PWV) along with several other derivatives for cardiovascular parameters, including heart rate and blood volume changes in arteries, which correlate with the blood pressure.
The system will incorporate a hardware design to localize the underlying vascular system of the body and focus on arterial sites for enhanced accuracy. The device will include a motion sensor to take into account the user’s movements, and the contact quality and reliability of the measurements. Advanced machine learning techniques, leveraging both general and personalized models, will be developed to convert bioimpedance measurements to blood pressure.
Researchers hope to validate the system and analytics in both a healthy patient cohort and a hypertensive cohort, learning the impact that nocturnal nondipping hypertension and anti-hypertensive treatments have on PWV and other correlated cardiovascular and blood pressure estimates.
Jafari said that while they have been working on their device for the past three years with a previous NIH grant, with the new funding the team will begin two new phases of the project at Texas A&M, where they will create novel techniques and methods, refine their prototype design and continue testing it.
“So the next version will be fully wearable and effectively would look like a smartwatch,” Jafari said. “Once we have that, we are going to start the evaluation of that technology on a cohort of human subjects.”
The last phase of the project will be at the Yale School of Medicine, where they will do extensive testing on hypertension patients who are on different medications. Eventually, their hope is that after decades of relying on the inflatable cuff-based technique, this new system could represent a significant change in how blood pressure is measured.
“We still have more battles to fight, but I think the likelihood of success at this point is very high,” Jafari said. “Our objective actually is to build a system that can work for anyone, even somebody who has absolutely no problem with blood pressure, but the ultimate objective is to enable anybody and everybody to have this kind of technology at their disposal.”
Other collaborators on the project include Dr. Harlan Krumholz and Dr. Erica Spatz, Yale School of Medicine; Melissa Grunlan, Department of Biomedical Engineering; Tom Ferris, Department of Industrial and Systems Engineering; and Bobak Mortazavi, Department of Computer Science and Engineering.
This article by Deana Totzke originally appeared on the College of Engineering website.