Center For Phage Technology Spearheads The Battle Against ‘Superbugs’
Ryland Young, director of the Texas A&M University Center for Phage Technology (CPT) in College Station, answered a call for help from a complete stranger in 2016. Consequently, he helped set in motion the advancement of phage therapy, a treatment for multi-drug resistant superbugs that could help the world avert a looming health care crisis. Researchers at the Navy Medical Research Center in Silver Spring, Maryland, answered the stranger’s call soon after.
Once in a while, events unravel in such a way that those involved are left with the distinct impression that much more than meets the eye is at work in the world. This was one of those occasions. Strangers living in three different cities, each located 1,400 miles away from the next, spaced evenly across the United States, were brought together to miraculously save the life of one man.
“Antimicrobial resistance is the number one health problem facing the world right now,” Young said. “The problem with antibiotics is that we’ve used them so much, and in some cases, abused them and overprescribed them, that now most serious bacterial pathogens, the bad bacteria, are resistant to drugs.”
The call
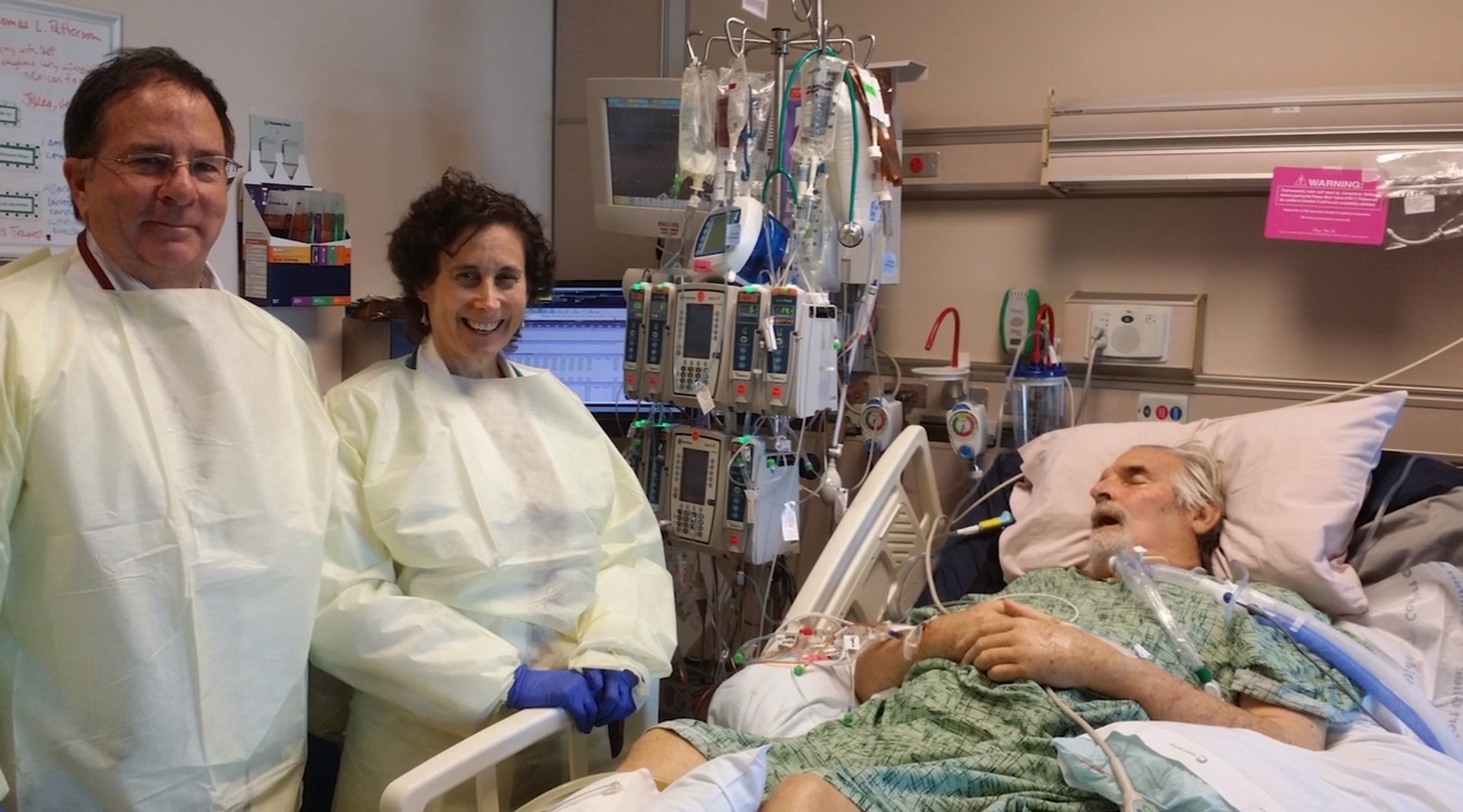
The call came from Steffanie Strathdee, a University of California-San Diego (UCSD) infectious disease epidemiologist, who was frantically searching for alternatives to antibiotics. Her husband, Tom Patterson, a UCSD psychiatry professor, had contracted a multi-drug resistant superbug on an Egyptian vacation, and doctors had exhausted every health care intervention available. His vital organ systems were failing, and he was comatose and dying at the UCSD Hospital. When Strathdee asked him if he wanted to live, he squeezed her hand.
“I thought it was God’s cruel joke at first, because here I was an infectious disease epidemiologist, and my husband was dying from an infection,” Strathdee said.
She found phage therapy, and though she remembered learning about bacteriophages decades earlier, she knew nothing about their therapeutic uses. Her online searches kept bringing up Young and the Texas A&M CPT, the only center of its kind for basic and applied phage research in the nation. Since 2010, the CPT has worked to resurrect phage technology.
Phages, viruses that grow on bacteria in nature, were abandoned as a potential treatment for bacterial infections when antibiotics became ubiquitous in the United States during World War II. Meanwhile, in other areas of the world, especially where antibiotics are unaffordable and inaccessible, doctors have continued developing and practicing phage therapy.
As Patterson lay at the brink of death, one of Young’s graduate students, Adriana Hernandez, and a recent graduate and lab technician, Jacob Lancaster, agreed to drop everything to help him. For three months, they worked day and night, literally around the clock, to provide Patterson with emergency phage therapy, and their last-ditch effort helped to save his life.
“It was 4 a.m. when I finally took my gloves off. I was listening to Tom Petty, and I was going to change the music because I needed something more upbeat. I was falling asleep,” Hernandez said. “I saw an email that Tom [Patterson] woke up, and I grabbed my phone in one hand, it was just baffling, ‘It worked, it worked,’ and I started to cry.”
Young and his team of researchers are working now to develop methods to safely, quickly and effectively harness and unleash the killing power of phages on multidrug-resistant bacteria infecting humans around the world. Beyond the science, they also are exploring best ways to tackle practical challenges such as securing FDA approvals and delivering the highly individualistic biological therapy, which is unlike the mass-produced antibiotics used most of the last century.
Ultimately, the goal of the CPT is to help develop the technology, standardize optimal delivery procedures and secure necessary approvals from regulatory agencies to make the treatment widely available to patients in the United States. The prominence of the Patterson case is helping to increase attention and funding for phage therapy in the United States.

“I thought it was God’s cruel joke at first, because here I was an infectious disease epidemiologist, and my husband was dying from an infection.”
Are superbugs responsible for more deaths in the U.S. than reported?
Generally, the United States and Western Europe address public health issues, such as potential bacterial epidemics, through improved procedures that are not always possible in other areas of the world. They avoid overcrowding, ensure high sanitation rates and carefully monitor food supplies.
At this time, most Americans who contract life-threatening bacterial infections pick them up in hospitals when they have major operations, Young said. Yet, in the United States alone, thousands of patients infected with superbugs die each year. Except in rare instances, experimental phage therapy is unavailable to them.
“How many others are going to be like Strathdee?” Young asked. “The surest way to get phage therapy, at this point, is to have 1,500 publications between you, your spouse and your physician.”
In a 2018 letter that was published in the journal of the Society for Healthcare Epidemiology, three doctors estimated that superbugs kill at least 153,000 patients each year in the United States. Their estimate, based on 2010 data, is almost seven times the number, 23,000, that is reported currently by the Centers for Disease Control and Prevention.
Strathdee believes the discrepancy is likely much larger now since the data that they used is almost a decade old. The doctors blame the difference, at least in part, on poor surveillance and reporting methods for multidrug-resistant bacterial infections.
According to a 2014 report, without effective health care interventions for superbugs, multidrug-resistant bacteria are expected to kill more people, 10 million, than cancer by 2050.
“I realized that even though I have training in infectious disease, that this whole global crises of antimicrobial resistance had snuck up on me,” Strathdee said. “That’s why we decided to tell our story. We’re considered the public face of superbugs these days.”
Jumping through bureaucratic hoops to fight infection
Strathdee turned to Dr. Robert Schooley, a UCSD infectious disease specialist and close family friend, with her desire to use experimental phage therapy to treat her husband, and he agreed to pursue the necessary emergency approvals from the FDA. However, he described challenges that Strathdee would have to overcome first.
Strathdee would need to find phages that were active against her husband’s superbug — the equivalent of finding the proverbial “needle in a haystack” — and ensure that they were prepared in a way that made them safe to administer to her husband.
She also would have to obtain approvals from two regulatory bodies, the institutional biosafety committee and the institutional review board, which monitor experiments at every major U.S. research institution, among others. And the legal teams of all involved parties would have to consent.
Only then could the phage therapy, which Schooley could not guarantee would work, be delivered to Patterson. And that was provided that Patterson would live long enough.
The frantic search for phages
In February of 2016, Strathdee sent an email message to every researcher she could find studying phage technology, and only Young responded that he would try to help.
The situation resonated with Young because Patterson was about his age, and Strathdee was an academic who spoke his language. And, nearing retirement, he wanted to see phage technology gain traction. He knew that saving Patterson’s life would be a game changer for the field.
“Texas A&M took a long-shot bet by investing in the center almost a decade ago because there was no evidence that all this would pan out,” Young said. “It’s going to turn out to be a good bet they made.”
Young called Strathdee and spent two hours explaining the processes necessary to find phages that would kill the particular strain of Acinetobacter baumannii ravaging her husband’s body.
Strathdee would have to send Patterson’s bacterial strain to his lab at Texas A&M, but first, she would have to obtain one of those approvals. Simply dropping a deadly bacterial strain in the mail was not an option.
“My feeling was that [all the necessary approvals] would take a long time, and it wasn’t consistent with the kinetics of Dr. Patterson’s illness,” Young said. “I hoped for the best, but I feared he was never going to live long enough to do all of that.”
The stars continued to align. Strathdee, a highly placed academic at UCSD, was able to cut through the bureaucratic red tape and expedite necessary approvals. She mobilized legal and regulatory representatives at her university to talk directly to representatives in the same positions at Texas A&M, and results that normally would have taken months took days.
“There were contacts at every level, side by side, and I think universities know how to work with each other,” Young said. “They do the same thing, the same way, so I think that’s what made it happen.”
Beyond petri dishes
In an unusual twist of events, researchers at Texas A&M, whose work generally is confined within walls of laboratories and to petri dishes, became intimately involved in providing life-saving medical intervention for Patterson.
Patterson’s bacterial strain arrived in College Station, and Young turned his lab into a central “command center.” He reached out to his contacts around the world to send whatever phages they had on hand to his lab for testing.
“Chip [Schooley] was very surprised that we were able to find a team that was willing to go to such lengths,” Strathdee said.
Hernandez and Lancaster put their lives on hold for two months to test the phages against Patterson’s superbug. In a race against time, they handed off a checklist at shift changes so that one could pick up easily where the other left off. Hernandez worked nights, and Lancaster worked days.
“I just literally said, ‘Let’s do this!’” Hernandez said. “I stopped everything I was doing, and I put my research on hold to focus all of my attention on getting phages, isolating phages, purifying phages [for the Patterson case].”
“I hoped for the best, but I feared he was never going to live long enough to do all of that.”
Testing and growing phages
To test phages against bacteria, the CPT researchers place media in flasks, add bacterial cells and wait. When the media becomes turbid with the bacteria, they add phages and wait again. They have their “needle in a haystack” when they return to find a crude product consisting of dead cell debris, including endotoxins from the bacterial cell coatings, and the phage cells that did the killing.
Phages are the only medicine that grows. For every one phage cell that kills one bacterial cell, 100 phage cells grow. This happens when a phage recognizes a receptor on the surface of the bacterial cell, attaches to the receptor, injects its DNA and multiplies. The bacterial cell explodes, releasing more phages to attack surrounding bacterial cells, and the cycle continues until 99 percent of the bacteria are dead. Each strain of bacteria has a different receptor, and likewise, each phage recognizes a different receptor.
The problem is that one mutant bacterial cell, meaning it has become resistant to the phage, is all it takes to eventually reverse any progress made by the phages. The solution for therapeutic and other applications, Young believes, is to treat each bacterial strain with a cocktail of three phages that recognize different bacterial receptors.
“There are not enough bacteria in the universe for resistance to emerge against a cocktail of three different phages,” Young said.
The command center
Hernandez and Lancaster began testing its small collection of A. baumannii phages and over 100 environmental samples already on hand at the CPT and those sent from labs around the world against Patterson’s superbug. They found three phages that killed the bacteria, but one of them could not reproduce to kill more bacteria.
As soon as the CPT found a phage that worked, Schooley began the process of getting special approval from the FDA. He learned during his conversation with the FDA that Navy researchers also were conducting phage research. They agreed to send whatever phages they could find to treat Patterson.
At the CPT, Hernandez and Lancaster isolated the phages from the crude product by spinning them two liters at a time in centrifuges that made 8,000 revolutions per minute.
“We broke two centrifuges in the lab by using them 24 hours a day for so long,” Hernandez said. “Actually, we burned up a centrifuge that Ry [Young] had used since he started his lab more than 40 years earlier.”
They then started the slow process of purifying the phages of debris and endotoxins that would be dangerous to Patterson, while they searched for more phages to kill his superbug. They collected new environmental samples from local sewage plants and animal pens and began testing them. Phages exist wherever bacteria are, so these places are obvious breeding grounds.
Meanwhile, Young looked for a faster way to clean the phages, and he contacted colleagues at San Diego State University who had streamlined a fast, safe and effective process and were willing to pitch in.
After that, the supply chain involved CPT isolating, propagating, concentrating and partially purifying the phages, which were then shipped to SDSU. There, Jeremy Barr in Forest Rohwer’s lab performed the final purifications and Anca Segall, another SDSU faculty member, assayed the final products and hand-delivered them to the UCSD Pharmacy. The first shipment arrived at SDSU at about day 10 and were processed and delivered to the hospital the next day.
“With Patterson, we were in such a hurry because we didn’t know from day to day if he was going to be alive,” Young said. “We found phages we knew worked and sent them as quickly as possible.”
On Tuesday, March 15, Schooley introduced the CPT phages into Patterson’s abdominal cavity where the infection started. Two days later, he injected the Navy’s phages into Patterson’s bloodstream. By Saturday, Patterson had emerged from his coma.
“To go from a deep coma where he was in what’s considered multisystem organ failure, where his lungs were failing, his heart was failing, and I had just signed a consent form for kidney dialysis — he was literally hours away from dying before we administered the first phages — to waking up, lifting his head off the pillow and kissing his daughter’s hand,” Strathdee said. “It was just mind blowing, not just for me and our family, but for the whole ICU.”
Later, Young and the Navy researchers learned that all the phages they hastily found and sent to Patterson had the same receptor. With the proper amount of time for testing, they would have sent a cocktail of phages with three different receptors.
“We were lucky in his case, because even though resistant bacteria did emerge quickly, his immune system took over when we lowered the overall load of bacteria in his body,” Young said.
Before administering the treatment, Schooley had conversations with members of the medical team in ICU at the UCSD Hospital who thought it strange to use experimental phage therapy on Patterson.
“It’s not that strange to do something that you don’t think will hurt, and you hope will help,” Schooley told them. “And once we had that discussion, other physicians came on board, and when they began to see him get better, many became true believers.”
What seemed a cruel joke to Strathdee at first has become an impetus for advancing phage research and funding that can save many more lives in the future. All involved in the Patterson case want to see phage therapy become accessible to any patient in need of the treatment.
Advancing phage technology in the lab and the classroom
The Texas A&M CPT is comprised of six laboratories that continue to conduct basic and applied phage research. The center employs a combination of 30 paid faculty, graduate students, post-docs and technicians. Students enrolled in phage courses in the Department of Biochemistry and Biophysics also contribute to the center.
Young interviews and selects 20 students each spring semester for his class, BICH 464: Bacteriophage Genomics, which provides students an opportunity to isolate and annotate the genomes of new phages.
All of the labs conduct fundamental research exploring the DNA structures of phages and how they work. Until the Patterson case, the center focused its efforts on applications for agriculture and industry. For example, one of the labs developed a phage cocktail to combat Pierce’s disease, a severe bacterial infection that has devastated grapevines in California. The center’s original charter focused on agricultural and veterinary applications because the regulatory burden for pursuing therapeutic uses in humans seemed too high.
In 2018, the UCSD launched the Center for Innovative Phage Applications and Therapeutics (IPATH). Strathdee established the Thomas L. Patterson Graduate Fellowship to support one doctoral student annually at the Texas A&M CPT, and Hernandez was the first recipient.
The road ahead for treating bacterial infections
Patterson’s case is helping researchers think about rational rules and regulations for developing and administering phage therapy. Different schools of thought about how the therapy should progress are being bounced around because the technology is still so young.
Young envisions phage therapy being delivered primarily as a service in hospital settings rather than in pill form in doctor’s offices. In his opinion, the first step in moving the treatment to the general public is developing libraries of phages that kill particular deadly bacteria. He believes that collections of 100 or so phages for each bacterium are necessary because every bacterial strain is different. Unlike antibiotics that work against all bacteria, one phage typically kills only one, two or a few strains of the same bacteria.
And phage therapy is single use, meaning phages will work only once against particular bacteria because they become resistant. Other phages must be used on the same bacteria the next time. The good news is that more phages exist in nature than any other organism, including bacteria, so the supply is virtually limitless.
In the future, hospitals might hire laboratories to fulfill orders for phages, but whatever the case, new technology is necessary, Young said. Doctors need ways to rapidly identify useful phages and target the bacteria. Rather than storing large doses of 100 phages needed for each bacterium, which would be expensive, Young speculates that keeping small doses of each and finding ways to rapidly amplify them might make better sense.
Young sees phage therapy becoming an important arrow in the quiver for treating bacterial infections, especially extreme cases, but he also envisions antibiotics making a comeback.
“I’m on the leading edge of the silver tsunami, that’s my generation, and we are likely going to see a lot more bad outcomes in hospitals because of antimicrobial resistance problems,” Young said. “Once significant numbers of Americans and Western Europeans start going into hospitals for knee and hip replacements and expiring because of drug resistance, there’s going to be a public outcry, and drug companies will start making antibiotics again.”
Overcoming regulatory barriers
Pharmaceutical companies have stopped developing antibiotics because they have become progressively more difficult to find and economically impractical to produce with the rapid rise of antimicrobial resistance. They also are not pursuing phage therapy because they have not yet determined ways to commercialize and monetize the treatment.
Antibiotics are chemical compounds that drug companies own for the period of the patent. They are intellectual property, and they are easily mass-produced, stored, dosed and distributed.
Conversely, phages are biologicals that companies cannot patent because they are found in nature. Phage therapy is personalized medicine, so every phage or phage cocktail is different. Purifying, growing and refrigerating all of them on a mass scale would be expensive and impractical.
Furthermore, all drugs used in the United States must meet good manufacturing practice (GMP) standards to be approved by the FDA. Rigorously testing antibiotics is necessary because chemical compounds can indiscriminately affect hundreds of types of tissue throughout the human body, and some of those effects might be harmful. Clinical trials are conducted exactly the same way using the same materials every time, or the results are considered “snake oil.”
As biological medicines, phages are not easy to produce to GMP standards, and conducting clinical trials on every phage in a large library would be impractical and prohibitively expensive. However, Young argues that purified phages meeting certain criteria do not need the same rigorous testing as new antibiotics, as phages are everywhere in nature and the risk of them causing direct harm to people is very low. Rather, he believes they need special FDA rules.
Purified phages cannot infect the human body. Unlike antibiotics, only two outcomes are possible with phages, and neither is harmful. The phages either work or they do not work. When they recognize dangerous bacteria, they rid the body of them. When they do not recognize the bacteria, they simply bump around each other until the body excretes them.
“The FDA is one of our most successful agencies,” Young said. “Very few people are complaining about the FDA, which means they haven’t made a whole lot of mistakes, so I believe they need to keep doing their job, but make special exceptions for phages.”
Media contacts:
- Sam Peshek, Texas A&M University Division of Marketing & Communications, peshek@tamu.edu.
- Ryland Young, CPT Director, ryland@tamu.edu.
- Mei Liu, CPT Program Director, meiliu@tamu.edu.