Doctoral Student Finds Alternative Cell Option For Organs-On-Chips

Organ-on-a-chip technology has provided a push to discover new drugs for a variety of rare and ignored diseases for which current models either don’t exist or lack precision. In particular, these platforms can include the cells of a patient, resulting in patient-specific discovery.
As an example, even though sickle cell disease was first described in the early 1900s, the range of severity in the disease causes challenges when trying to treat patients. Since this disease is most prevalent among economically poor and underrepresented minorities, there has been a general lack of stimulus to discover new treatment strategies due to socioeconomic inequity, making it one of the most serious orphan conditions globally.
Tanmay Mathur, doctoral student in Abhishek Jain’s lab in the Department of Biomedical Engineering at Texas A&M University, is developing personalized blood vessels to improve knowledge and derive treatments against the vascular dysfunction seen in sickle cell disease and other rare diseases of the blood and vessels.
Current cells used in blood vessel models use induced pluripotent stem cells (IPSCs), which are derived from a patient’s endothelial cells. However, Mathur said these cells have limitations — they expire quickly and can’t be stored for long periods of time.
Mathur’s research offers an alternative — blood outgrowth endothelial cells (BOECs), which can be isolated from a patient’s blood. All that is needed is 50 to 100 milliliters of blood.
“The equipment and the reagents involved are also very cheap and available in most clinical settings,” Mathur said. “These cells are progenitor endothelial cells, meaning they have high proliferation, so if you keep giving them the food they want, within a month, we will have enough cells so that we can successfully keep on subculturing them forever.”
However, the question is do BOECs work like IPSCs in the context of organ-on-chips, a microdevice that allows researchers to create these blood vessel models. That’s a question Mathur recently answered in a paper published in the Journal of the American Heart Association.
“By combining the analytics of our vessel-chip with next-generation RNA sequencing, I was able to show that BOECs do not differ in any statistical way compared to the other cells,” Mathur said. “Not only can you do patient-specific studies with BOECs, you can also use these cells as alternatives to existing cells, because at the end of the day they are still primarily human cells. If there is a way for you to get patient-derived cells with the least effort possible, that’s always the best way to move forward.”
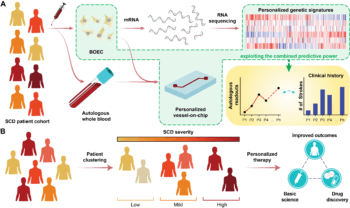
Mathur’s next step is to begin testing a larger cohort of blood samples with sickle cell disease and bring computation into the project through machine learning and artificial intelligence. By developing a chip model that can predict variables such as clotting time, inflammation and more, the algorithm can relate each patient’s history and treatment to their disease status.
“Say I made a model on 100 patients,” Mathur said. “If you give me the 101st patient and I run the same methodology for those cells, my algorithm should be able to predict if that patient is a severe, moderate or mild sickle cell patient. It’s of importance because the clinician wants to know what is the most effective short-term and long-term treatment strategies for the patient.”
“Tanmay’s work lays a foundation for what lies in the future of tissue engineering and organ-on-chip technology and the positive impact that these platforms can make in personalized medicine,” Jain said.
Developing this algorithm will help reduce the guesswork clinicians have to do in creating treatment plans.
“Right now, we do not know how much of a drug to give to a mild patient. That’s why we overcorrect or undercorrect for complications,” Mathur said. “Every drug will have certain side reactions, which you can only minimize. The best way to minimize is to tailor your therapy for each patient. I’m trying to minimize this iteration and minimize the cost as well as maximize the success of the therapy for the patient.”
This article by Jennifer Reiley originally appeared on the College of Engineering website.