A New Technique To Assess Differences Of Cancer Cells In Tumors
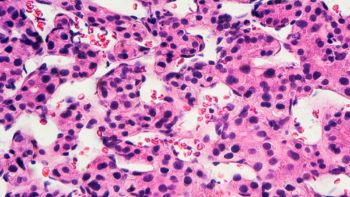
Not all tumors are made alike. A tumor may consist of different populations of cancer cells, each having its own distinct genetic and metabolic profiles. These differences create obstacles for effective cancer therapy because the drugs may suppress one group of cells but leave another intact.
A team of researchers from Texas A&M University and the California Institute of Technology (Caltech), are working together to develop a technique to help solve this problem.
Dr. Jun Zou, professor in the Department of Electrical and Computer Engineering, and his Ph.D. student Song Xu, worked with Dr. Lihong V. Wang, Ben Professor of Medical Engineering and Electrical Engineering, and his students at Caltech to develop a solution that would allow researchers to assess the level of difference in cancer cell metabolism inside a tumor.
Their technique could facilitate the design of effective and personalized treatment strategies by providing useful information for predicting tumor growth, invasion and drug resistance.
To assess the metabolic state of the cells, the team looked at what fed them, hemoglobin. This natural oxygen carrier and supplier undergoes a significant color change when it adsorbs or desorbs oxygen. This unique property makes hemoglobin an ideal biocompatible optical-absorption-based oxygen sensor. The oxygen consumption rate (OCR) of a cell (the amount of oxygen consumed by a cell in a certain amount of time) is directly related to its metabolic state. Therefore, measuring the OCRs of single cancer cells will provide a good picture of their metabolic profiles.
The team then combined this process with photoacoustic microscopy, an imaging technology where laser light produce ultrasonic vibrations in a sample. Researchers can use the vibrations to detect changes in optical absorption of cells, blood vessels and tissues. They were able to monitor tiny variations in oxygen saturation of hemoglobin, from which the OCR of a single cell trapped and sealed inside an airtight microwell can be calculated. They call this technique single-cell metabolic photoacoustic microscopy (SCM-PAM). Combining with a unique microfabricated single-cell microwell array they developed, SCM-PAM can be scaled up to measure a large population of single cells, which would be needed for testing the variety of cell populations in a tumor.
“With its unique capability for label-free, high-throughput single-cell OCR measurement and the potential of providing multidimensional information about tumors, we believe SCM-PAM will become a useful tool for both fundamental cancer research and clinical personalized cancer therapy,” Zou said.
Using their technique, the team demonstrated in a recent paper published in Nature Biomedical Engineering that their technology could review about 12,000 cells per hour, 100 times higher than current techniques limited to about 120 cells per hour.
Funding for the research was provided by the National Science Foundation and the National Institutes of Health.
This article originally appeared on the College of Engineering website.